
Under the framework of Good Manufacturing Practice (GMP), the air filtration system has become a core engineering element in the pharmaceutical industry to ensure the sterility of drugs and prevent cross-contamination. With the rapid development of biopharmaceutical and cell therapy technologies, air filtration technology is evolving from traditional particulate control towards microbial interception, molecular contaminant removal, and intelligent monitoring.
I. Grading of Pharmaceutical Clean Environments and Regulatory Requirements
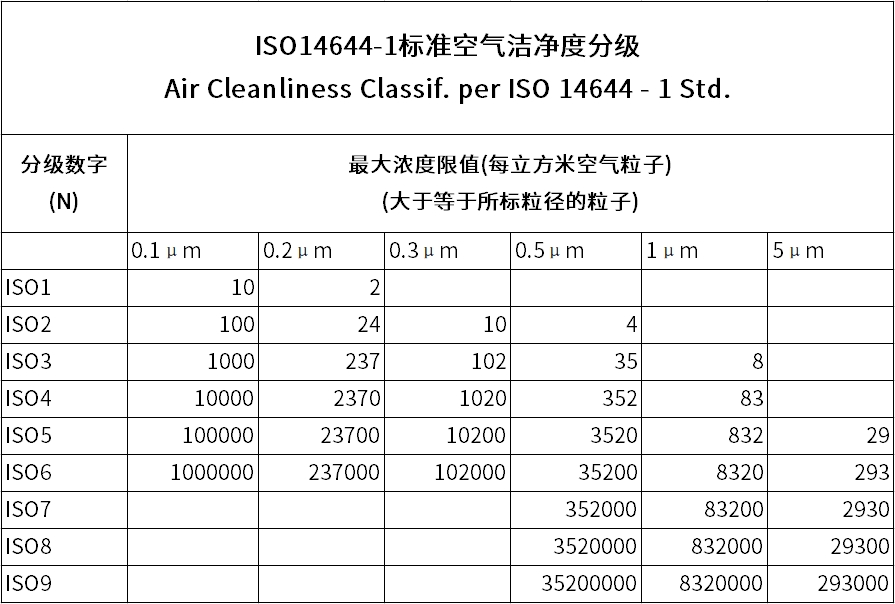
1. International Standard Systems
- ISO 14644-1: In Class A areas, the requirement is that the number of particles ≥ 0.5μm is ≤ 3520/m³ (static state).
- EU GMP Annex 1: It is specified that the microbial limit in Class A areas should be ≤ 1 CFU/m³.
- FDA 21 CFR 211: It is compulsorily stipulated that the HVAC (Heating, Ventilation and Air Conditioning) system should have the ability of dynamic pollution control.
2. Key Control Parameters
- Inactive Particle Control: Monitor the gradient distribution of particle sizes from 0.5-5μm.
- Active Particle Interception: Use an airborne bacteria sampler for dynamic microbial detection.
- Differential Pressure Gradient Design: The differential pressure between the core area and the adjacent areas should be ≥ 15Pa (dynamically maintained). Taking the production of lyophilized powder injections as an example, the Class A area of the filling line needs to meet the following simultaneously:
- Suspended Particles: ≥ 0.5μm ≤ 20 particles/m³ (dynamic state).
- Settling Bacteria: ≤ 1 CFU/4 hours (φ90mm Petri dish).
- Surface Microorganisms: ≤ 3 CFU/contact dish (glove surface).
II. Architecture of Pharmaceutical-specific Air Filtration Systems
1. Optimization of the Three-stage Filtration System
- Primary Filtration Stage: G4/F8 grade synthetic fiber filter media, intercepting particles ≥ 5μm (ASHRAE 52.2 standard).
- Medium-efficiency Filtration Stage: H13-H14 grade HEPA (High Efficiency Particulate Air) filters, with an efficiency of ≥ 99.97%-99.995% for 0.3μm particles.
- Terminal Filtration Stage: U15 grade ULPA (Ultra Low Penetration Air) filters (interception rate for 0.12μm ≥ 99.9995%), used for Class A laminar flow hoods.
2. Microbial Control Technologies
- Hydrophobic Coating Treatment: PTFE (Polytetrafluoroethylene) coated filter media with a contact angle > 130° to prevent the growth of microorganisms in humid environments.
- Sterilization Compatibility: The filter can withstand steam sterilization at 121℃ for ≥ 50 times (EN 1822 certification).
- Integrity Test: Use DOP/PAO (Dioctyl Phthalate/Polyalphaolefin) aerosol scanning to ensure that the local leakage rate < 0.01%.
3. Chemical Contaminant Control
- Activated Carbon Adsorption Module: Coconut shell activated carbon with an iodine value ≥ 950mg/g, and the adsorption capacity for benzene series compounds > 30%.
- Molecular Sieve Treatment Unit: 3A type molecular sieve to control the water activity (Aw < 0.6).
- Catalytic Oxidation Device: Precious metal catalyst to achieve a VOCs (Volatile Organic Compounds) removal rate > 90% (under the condition of 250℃).

III. Analysis of Applications in Typical Pharmaceutical Scenarios
1. Production Area of Sterile Preparations
- RABS (Restricted Access Barrier System)/Isolator System: Equipped with a double-layer HEPA air supply unit, with an air change rate > 100 times per hour.
- Laminar Flow Hood of the Filling Line: The wind speed is maintained at 0.45m/s ± 20% (ISO 5 class standard).
- Sterilization Tunnel: The temperature resistance of the filter in the high-temperature section > 350℃ (316L stainless steel frame).
2. Biological Fermentation Workshop
- Intake Air Treatment: Absolute filter (0.01μm PTFE membrane) to achieve the supply of sterilized air.
- Tail Gas Treatment: Two-stage deep filters (glass fiber + borosilicate) to prevent the leakage of bacteria.
- Phage Prevention and Control: 0.1μm ultrafiltration membrane combined with an ultraviolet photocatalytic inactivation system.
3. Production Area of Solid Preparations
- Dust Removal System: Pulse reverse blow bag-in-bag-out (BIBO) design, with a dust interception efficiency > 99.99%.
- Cross-contamination Prevention and Control: Special exhaust air filter (H14 grade + activated carbon) to handle highly allergenic materials.
- Humidity Control: The dew point temperature of the rotary dehumidifier ≤ -40℃ (API production area).

IV. Key Technical Challenges and Breakthroughs
1. Control of Microbial Regeneration Risks
- Develop a composite antibacterial coating of nano-silver/zinc oxide (bacteriostasis rate > 99.9%).
- Implement online electrochemical impedance spectroscopy (EIS) to monitor the formation of biofilms.
- Periodic atomization fumigation with hydrogen peroxide (6log microbial killing effect).
2. Adaptability to High Humidity Environments
- Increase the contact angle of hydrophobic filter media to 150° (ASTM D7334 standard).
- Develop a gradient density glass fiber structure (dust holding capacity increased by 40%).
- Intelligent condensate water drainage system (humidity sensor accuracy ± 1.5%RH).
3. Intelligent Operation and Maintenance System
- Differential pressure sensor network (accuracy ± 1Pa) to monitor the filter resistance in real time.
- Digital twin system to predict the service life (error < ± 5%).
- Blockchain technology to make the filter replacement records unalterable (compliant with FDA 21 CFR Part 11).
V. Trends in the Development of Cutting-edge Technologies
- Self-sterilizing Filter: Visible light catalytic materials (such as g-C3N4/TiO2) to achieve in-situ microbial decomposition.
- Ultra-low Leakage Technology: Laser welding frame structure reduces the frame leakage rate to 0.001%.
- Application of Intelligent Materials: Temperature-sensitive shape memory polymers to achieve dynamic adjustment of pore sizes.
- Continuous Monitoring System: Microfluidic chip technology to achieve microbial detection at the single-cell level.

According to industry forecasts, the pharmaceutical air filtration market will grow to 3.7 billion US dollars by 2028 (with a Compound Annual Growth Rate (CAGR) of 6.8%), among which the demand for biosecurity filters will increase by 12%. With the deepening of the QbD (Quality by Design) concept, the air filtration system is transforming from a cost center to a core guarantee element for drug quality.



















